R Lobectomy
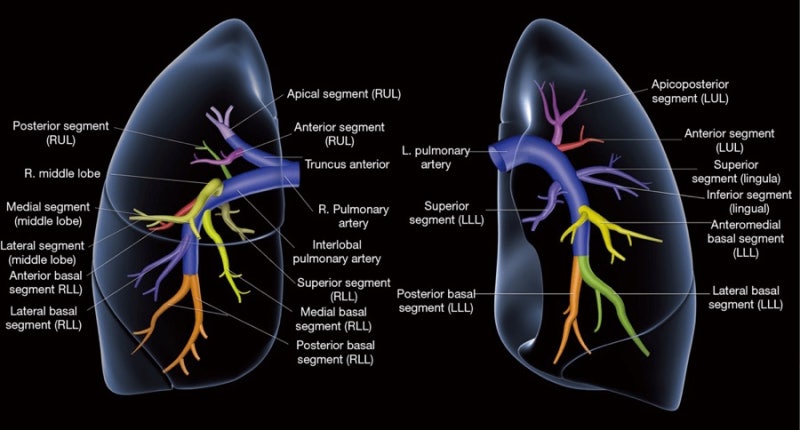
Video-assisted versus open thoracotomy lobectomy: comparison on lymphadenectomy and survival in early stage of lung cancer Dariusz A. Dziedzic 1^, Marcin Zbytniewski 1^, Grzegorz M. Gryszko 1^, Marcin M. Cackowski 1^, Renata Langfort 2, Tadeusz M. Conventional treatment of early-stage non–small cell lung carcinoma continues to be anatomical lobectomy with systematic mediastinal lymphadenectomy. A lobectomy is a generally safe procedure that treats more serious forms of different lung diseases. Thankfully, there are also options for robotic lung surgery which are less invasive and can reduce your recovery time. Learn when to seek a lobectomy and how this treatment treats each lung problem. Lung lobectomy is a type of thoracotomy surgery done to remove one or more lobes of your lung. Your lungs are two hollow organs that are covered by pleural sacs (two thin layers of tissue). Each lung is divided into lobes (sections) by deep grooves. Normally, your right lung has three lobes while your left lung has two lobes.
Introduction
Although non-small cell lung cancer (NSCLC) usually requires multi-modality therapy that includes systemic chemotherapy and/or radiation therapy, surgical resection is still standard for diagnosis, treatment, and staging. Mediastinal lymph node (LN) status is the most important prognostic factor after surgical resection of early stage NSCLC (1). Patients with positive LNs at surgical resection are offered adjuvant chemotherapy and/or radiation therapy.
Minimally invasive robotic-assisted surgery is one of recent technologic developments that are advancing thoracic surgery. Robotic surgical systems, such as the da Vinci® (Intuitive Surgical Corporation, Sunnyvale, CA, USA), provide surgeons a handful of advantages, including high-definition, magnified, three-dimensional views of the operating field, wristed instrumentation with multiple degrees of articulation, and computer-assisted scaled-down movements and reduction of hand-related tremors, which make hilar and mediastinal dissection more precise. These capabilities are expected to allow more effective LN dissection, resulting in improved detection of clinically occult locoregional metastases.
Benefits of robotic-assisted surgery continue to be debated. One lingering criticism of minimally invasive surgery (MIS), such as conventional video-assisted thoracoscopic surgery (VATS), is that mediastinal LN dissection mediastinal lymph node dissection (MLND) is inadequate when compared with that by thoracotomy (2). Thus, the proportion of lobectomies performed by MIS approach are lower than what we would expect more than two decades after the first VATS lobectomy in 1991, with proportions of lobectomies performed by conventional VATS estimated to be as low as 6% in the Nationwide Inpatient Sample database and as high as only 32–45% in the highly selected Society of Thoracic Surgery General Thoracic Surgery database (3-6). Furthermore, a study using data from eight states compared distribution and outcomes between different lobectomy approaches, and only 430 cases (1.3%) were performed robotically, although this percentage increased from <1% in 2008 to 3.4% in 2010 (7).
Current guidelines recommend ≥3 ipsilateral mediastinal (N2) LN stations assessed in addition to removal of regional N1 LN stations for appropriate surgical staging of NSCLC. Our primary objective in this study was to investigate whether robotic-assisted VATS (R-VATS) surgery improves overall LN dissection and LN metastasis detection during pulmonary lobectomy for NSCLC.
Methods
We retrospectively analyzed prospectively collected data from all patients who underwent any thoracic surgical procedure at our institution by one surgeon. For this study, we included consecutive patients who underwent R-VATS lobectomy, including those converted to open lobectomy, from September 2010 through August 2013. From these patients, we analyzed those with NSCLC on final pathology.
This study was conducted in accordance with the amended Declaration of Helsinki as outcomes research for quality assurance as part of our departmental thoracic oncology clinical research database protocol. This database protocol was approved by our institution’s Scientific Review Committee (MCC #16512) and our university’s Institutional Review Board (IRB #Pro00002678), which waived informed consent for this retrospective study, which is considered as review of existing data. Nevertheless, all patients gave informed consent for our standard surgical procedure, which consists of fiberoptic bronchoscopy, R-VATS lobectomy, or else R-VATS wedge resection followed by completion lobectomy, and then MLND, with possible thoracotomy. Some patients also gave informed consent for any anticipated en bloc chest wall and/or vertebral resection, with possible reconstruction. Through our institutional surgical informed consent, patients gave permission to use surgery-related and tissue-related data for education and research purposes.
All our patients undergo fiberoptic bronchoscopy by the operating surgeon after the induction of general anesthesia. After placement of the dual-lumen endotracheal tube, the patient is then placed in either right or left lateral decubitus position, depending in which hemithorax the lesion is located. Our robotic-assisted lobectomy technique utilizes a three-port system, which includes a 4-cm camera port along the 6th intercostal space (ICS) at the anterior axillary line, which doubles as the assistant’s access port, and two 1-cm instrument ports along the 3rd ICS at the anterior axillary line and along the 9th ICS at the posterior axillary line. This 3-port anterior approach is adapted from our 2-port approach for conventional VATS lobectomies, which uses a 1-cm camera port in the 8th or 9th ICS at the posterior axillary line and a 4-cm instrument port along the 5th or 6th ICS at the anterior axillary line and which allows use of the thoracoscope in either port. Since a 4-cm incision is ultimately required to deliver the resected lobe with the lung cancer from the thoracic cavity, we have not adopted a totally port-based approach. Our 3-port anterior approach differs from that of Park and colleagues only in the choice of ICS for the port incisions (e.g., the 3rd, 6th, and 9th ICS for our port incisions instead of the 4th, 7th, and 10th ICS for theirs) and the choice of the port which is shared by the assistant for access (e.g., our assistant sharing the 6th ICS camera port incision instead of their assistant sharing the 4th ICS instrument port incision) (8). Based on our three port incisions, the robotic patient cart is docked behind the patient and over the patient’s ipsilateral shoulder, with alignment of the robotic patient cart’s center post, the patient’s scapular tip, and the camera port at the 6th ICS along the anterior axillary line.
From September 2010 through December 2011, our group used the da Vinci S™ Robotic Surgical System, with the Si™ system being used from January 2012 to the present. The lobectomy is performed with the pulmonary vein divided first, then the pulmonary artery branch(es) and bronchus, and then completion of the pulmonary fissures. While we have not needed to use the fourth arm of the robotic patient cart, we have created a fourth port, usually along the 10th ICS at the mid scapular line, on rare occasion to allow for another angle from which to apply the linear endostapler onto a difficult pulmonary artery branch, particularly when performing a left upper lobectomy. After delivery of the lobectomy within an endopouch through the 6th ICS port incision, complete MLND is then performed. We prefer to have our assistant use a “sponge stick” to retract the lung and expose the mediastinal LN stations, rather than to use the fourth arm of the robotic patient cart, in order to simplify the robotic patient cart set-up and docking and to minimize risk of both internal and external collisions between the robotic patient cart arms. At the end of the procedure, a 32-French chest tube is introduced through the 9th ICS port incision and connected to drainage at −20 cm H2O continuous suction.
Our exclusion criteria selected out patients with pathology other than NSCLC, such as benign lesions, pulmonary metastasis, or small cell carcinoma, and cases that resulted in conversions to pneumonectomies. For this study, we also excluded patients who received neoadjuvant chemotherapy and/or radiation therapy, as neoadjuvant treatment would downstage LNs that have metastatic carcinoma, while neoadjuvant radiation therapy may make LNs appear clinically positive on preoperative positron-emission tomography (PET) scan.
Variables and outcomes analyzed included demographics, intraoperative estimated blood loss (EBL), operative time (skin incision to skin closure), conversion to open lobectomy, chest tube duration, hospital length of stay (LOS), and in-hospital mortality. All clinically significant perioperative complications were noted. Respiratory complications included acute respiratory failure, pneumonia, aspiration confirmed by imaging, hypoxia requiring home oxygen, prolonged air leak lasting ≥7 days, mucous plugs, subcutaneous emphysema, pneumothorax after chest tube removal, effusion requiring intervention, chyle leak, pulmonary embolism, and postoperative hemothorax requiring intervention. Cardiac complications included atrial fibrillation, other arrhythmia requiring medications, myocardial infarction, and cardiopulmonary arrest. Other complications evaluated were other infections and multiorgan system failure and/or shock.
Clinical stage was determined by history and physical, computerized tomography (CT) scan, PET scan, brain imaging studies, endobronchial ultrasonography (EBUS), and/or cervical mediastinoscopy. LNs with short-axis diameter greater than 1 cm on CT scan or maximum standardized uptake value (SUVmax) greater than 2.5 on PET scan were considered clinically positive. Otherwise, CT scans, PET scans, and brain imaging studies were mainly used to rule out stage-IV disease. We did include in this study one stage-IV patient with isolated brain metastasis and who subsequently underwent pulmonary lobectomy for their primary lung cancer. At our institution, we do not routinely perform cervical mediastinoscopy for patients who are candidates for pulmonary lobectomy, even those who have biopsy-proven to have, or are suspected as having, clinical stage-IIIA NSCLC. These patients routinely undergo surgical resection, followed by adjuvant chemotherapy if proven to have pathologic stage-II NSCLC or by adjuvant chemotherapy and radiation therapy if proven to have pathologic stage-IIIA NSCLC. For patients who are suspected as having clinical stage-IIIB NSCLC or who may require pneumonectomy for curative resection, EBUS-guided fine needle aspiration (FNA) of mediastinal LNs is performed to confirm N3 or N2 disease, respectively. Transthoracic or transbronchial biopsy of a lung mass with or without EBUS-FNA of mediastinal or hilar LNs is also performed to confirm diagnosis for unresectable lung cancers. Cervical mediastinoscopy is performed if suspicion for N2 or N3 LN involvement is high, but EBUS-FNA is inconclusive.
Pathologic stage was based on intraoperative findings and final pathology. Tumor histology, tumor size, and numbers and locations of all LN stations and of all individual LNs were also analyzed. We routinely performed systematic hilar and mediastinal LN dissection, which included LN stations 2R, 4R, 7, 9R, 10R, and 11R, and occasionally stations 3A, 3P, and 8R for right lobectomies and LN stations 5, 7, 9R, 10R, and 11R, and occasionally stations 6 and 8R, for left lobectomies. Except during segmentectomies, we did not perform LN dissection at stations 12 or higher and instead deferred examination of these stations to the pathologists. We then compared clinical and pathologic tumor, nodal, and metastasis (TNM) stage.
Data were presented as mean or median, with standard error of the mean (SEM) and range, or as count and percentage, unless otherwise specified. Where applicable, we used Chi-square (χ2), Fisher’s exact test, or Student’s t-test to compare variables, with statistical significance established at P≤0.05.
We then performed an extensive literature review on LN dissection and upstaging in thoracotomy and conventional VATS series. Due to the lack of available data about efficacy of robotic-assisted surgery on LN dissection and upstaging after pulmonary lobectomy for resectable NSCLC, our paper will attempt to address these endpoints. By “resectable”, we refer to those stages of lung cancer, namely stage-I, stage-II, and stage-IIIA, including those with chest wall involvement, for which surgical resection can be offered for curative intent, even if adjuvant chemotherapy and/or radiation therapy is subsequently required. In our series, while patients with stage-IIIB and stage-IV NSCLC would be generally excluded, we did include two patients with clinical stage-IIIB, based on vertebral involvement, and one patient with clinical stage-IV, based on a solitary brain metastasis, all three of which were offered surgical resection with curative intent. Lastly, we included neuroendocrine tumors within our cohort, as these tumors were either carcinoids or large cell neuroendocrine tumors, which are treated by most medical oncologists as NSCLC, despite being classified as being in the same family as small cell lung carcinoma (SCLC), and which were able to be offered surgical resection with curative intent (9,10).
Results
Upper Right Lobe Removal
There were 211 consecutive patients, who underwent robotic-assisted pulmonary lobectomy between September 2010 and August 2013 by one surgeon. Eliminated from this initial cohort were 3 patients, who underwent conversion to pneumonectomy due to hilar tumor involvement that precluded lobectomy, and 17 other patients, who received neoadjuvant chemotherapy and/or radiation therapy. Final pathology for 32 patients reported benign lesions, pulmonary metastases, or SCLC. These exclusions left for evaluation our cohort of 159 cases. Mean age of our final cohort was 68±0.8 years (range, 39–86 years). Description of demographics and disease characteristics are included in Table 1.
Full table
Types of resections are reported in Table 2. The tumor was located in the right lung in 106 patients and in the left lung in 53 patients. The three most common anatomic locations were right upper lobe (42.1%), left upper lobe (24.5%), and right lower lobe (11.3%).
Full table
Major intraoperative outcomes included a median EBL of 150±26 milliliters and a median skin-to-skin operative time of 172±6 min. Overall conversion rate to open lobectomy was 8.8%, while the emergent conversion rate for bleeding control was 4.4%. Our patients’ median hospital LOS was 5±0.4 days. In-hospital mortality rate for our cohort was 1.9% (Table 3), with our three in-hospital deaths having occurred during our first 50 cases. Two patients died when their respective families requested withdrawal of support after prolonged courses of multiorgan system failure. The third patient died after a cardiac arrest.
Intraoperative and postoperative complications rates were 7.6% and 39.0%, respectively. The three most frequent postoperative complications in our series included prolonged air leak lasting ≥7 days, new-onset atrial fibrillation, and pneumonia (Table 3). We had a minor plus major respiratory complication rate of 32.1%, with prolonged air leak being the most frequent, occurring in 17.6% of our patients, followed by pneumonia (10.7%) and mucus plugging requiring bronchoscopy (8.2%).
The number of N2 LN stations assessed intraoperatively differed from the number of N2 LN stations with actual LNs reported by the pathologist (4.1±0.1 vs. 3.7±0.1 LN stations, respectively). We had a mean number of 7.2±0.3 individual N2 LNs retrieved. Our overall mean of N1 + N2 LN stations reported was 5.6±0.1 LN stations, with a total of 13.4±0.4 individual N1 + N2 LNs (Table 4).
Full table
Clinical and pathologic staging are compared in Table 1. Three-fourths of our cohort were clinical stage IA or IB preoperatively. Postoperatively, the number of patients with pathologic stage IA and stage IB was 60.4%, with the most common pathologic stage being IA. There was one clinical stage-IV patient with isolated brain metastasis that was resected prior to subsequent pulmonary lobectomy. Two clinical stage-IIIB patients had vertebral involvement that required vertebral resection en bloc with pulmonary lobectomy. Our overall upstaging rate was 30.2% (48/159). The upstaging rate from clinical N0 to pathologic N1 (cN0-to-pN1) and from cN0 to pathologic N2 (cN0-to-pN2) among our cohort was 8.2% and 8.2%, respectively (Table 5).
Full table
Discussion
With median skin-to-skin operative time of 172 min, median EBL of 150 milliliters, overall conversion rate of 8.8%, and emergent conversion rate of 4.4%, the major perioperative outcomes from our cohort are comparable with what have been previously described for robotic-assisted lobectomy as well as for conventional VATS and open lobectomy. Meta-analysis of currently available literature on robotic-assisted pulmonary lobectomy, including data from 326 patients, showed a pooled average operative time of 215 min and overall conversion rate of 9.4% (11). Lower conversion rates (i.e., 3.3%) to open thoracotomy have been published after R-VATS lobectomy for early stage NSCLC (12). However, we did not exclude patients with resectable advanced NSCLC stages from our cohort of patients, and bulky hilar lymphadenopathy did contribute to our overall and emergent conversion rates to open lobectomy.
Our 1.9% mortality rate and median hospital LOS of 5 days are also similar to what have been published. The meta-analysis of currently available literature on robotic-assisted lobectomy, showed a pooled mortality rate of 2.1% and hospital LOS of 6 days (11). A separate systematic review of R-VATS lobectomy literature reported overall morbidity rates from 10% to 39%, with perioperative mortality rates from 0% to 3.8% (13). Mortality rates reported in the literature for open and conventional VATS approaches are 2.5% and 2.1%, respectively (14).
As with conventional VATS lobectomy, R-VATS lobectomy has been associated with better postoperative outcomes, including less postoperative pain, shorter hospital LOS, and fewer complications, compared to open lobectomy. Despite MIS having been shown to be beneficial with respect to postoperative outcomes, only 30–45% of pulmonary lobectomies are being performed using conventional VATS. Limited visual field, 2-dimensional visualization, lack of articulating thoracoscopic instruments, lack of scaling down of movements, and non-intuitive instrument movements relative to the surgeon’s hand movements have contributed to the slow adoption of conventional VATS (15). Since the introduction of MIS, the debate regarding benefits, outcomes, and costs has been addressed in multiple studies. In 2009, Yan et al., (16) published a systematic review and meta-analysis of many of these studies and concluded that conventional VATS lobectomy for early-stage NSCLC may be a valid alternative to open surgery if the procedure is performed in qualified centers. Supporters of MIS now promote expanding the use of conventional VATS (17) and R-VATS (13) to special populations, such as to patients with advanced age (>70 years of age) and patients with pulmonary compromise. Many believe that R-VATS can be the new leading edge of the paradigm shift toward minimally invasive thoracic surgery.
These limitations of conventional VATS instrumentation contribute to an oncologic reason for the reluctance to adopt conventional VATS for lobectomies, which is that MLND may not be as adequate by MIS, such as by conventional VATS, compared to that by the open approach (18). However, R-VATS surgery may provide the precision necessary to perform meticulous hilar and mediastinal LN dissection. The data from our current study suggests that robotic-assisted surgery may help allay these concerns about MLND during MIS for NSCLC. From our cohort, 99.4% of the patients had at least 1 mediastinal LN station reported. In a study of >11.000 NSCLC patients treated surgically, most of them with open lobectomy, only 57.8% of patients underwent any MLND (19). Another review suggested that only 40% of the lobectomy/bilobectomy performed in the United States of America for lung cancer had documented mediastinal LN assessment (20).
Inability to assess some LN stations is commonly due to anatomic variation, possibility of combining anatomically adjacent LN stations as one LN station, inadequate instrumentation, inexperienced surgeons, and fear of increasing morbidity and/or mortality to the procedure. Watanabe, in one of his series, addressed this last concern by showing that dissecting 3–4 mediastinal LN stations did not add morbidity to the surgery. He reported a 3.5% intraoperative complication rate, including a 1.5% recurrent laryngeal nerve injury rate, 0.3% bilateral vagal injury rate, 1.4% chylothorax rate, and 0.3% airway injury rate (21), which are comparable to rates previously published by others.
Current guidelines suggest that assessment of 3 or more N2 LN stations is the most important prognostic factor in NSCLC staging (22). Of our robotic-assisted lobectomy cohort, 98.1% (156/159) reached this goal. Pathology confirmed that 88.7% (141/159) of our cases had more than three N2 stations dissected. In a feasibility study performed by Swanson and colleagues, 50% of patients who underwent conventional VATS lobectomies included only ≤2 N2 LN stations (23). Another series from the United Kingdom reported that only 40–60% of conventional VATS cases had ≥3 LN stations resected (24). D’Amico and colleagues analyzed the National Comprehensive Cancer Network (NCCN) NSCLC database and found that ≥3 mediastinal LN stations were assessed in only 66% of patients in the conventional VATS group and in only 58% of patients in the open group (25).
Preference of LN sampling versus dissection is dependent on the surgeon’s philosophy in terms of adequacy of LN sampling for oncologic staging, while completeness of LN dissection is dependent on the surgeon’s experience and skill in terms of LN dissection during a particular approach. We prefer to perform lobectomies via a minimally invasive approach over thoracotomy (95% vs. 5%) based on proven benefits of MIS, and we prefer to perform anatomic lung resections via the R-VATS approach over the conventional VATS approach (90% vs. 5%) due to the more precise hilar dissection and lower conversion to thoracotomy afforded by the robotic system and mediastinal dissection. During the same time period as for this study, we performed eight conventional VATS lobectomies without robotic-assistance. The number of mediastinal LN stations assessed and reported among our small cohort of conventional VATS lobectomy cases were 3.3±0.2 and 2.8±0.3 N2 LN stations, respectively, compared to 4.1±0.1 and 3.7±0.1 N2 LN stations, respectively, for our R-VATS cases. Also among our small cohort of conventional VATS lobectomy cases, a mean of 6.8±1.6 individual mediastinal LNs and a mean total of 11.8±1.6 individual N1 + N2 LNs were retrieved, compared to a mean of 7.2 individual N2 LNs retrieved and a total of 13.4 individual N1 + N2 LNs among our R-VATS cohort.
In the NCCN NSCLC database study, the mean number of N2 LN stations assessed by conventional VATS was 3.2 N2 LN stations and 2.9 N2 LN stations by the thoracotomy approach (25). In comparison, our mean total of 4.1 N2 LN stations assessed and 3.7 N2 LN stations reported with actual LNs dissected with R-VATS appears to be greater. While the number of nodal stations assessed may not differ significantly between R-VATS and conventional VATS approaches, this similarity may be due to there being only a small number of mediastinal nodal stations (2R, 4R, 3, 7, 8R, and 9R on the right; 5, 6, 7, 8L, and 9L on the left) that can be assessed. In contrast, the number of individual LNs assessed between R-VATS and conventional VATS vary more widely due to the comfort and preference of the surgeon and their technical ability (instrumentation and experience) to perform nodal sampling versus complete nodal dissection within confined mediastinal spaces.
A series of 543 cases from Denlinger and colleagues showed that the mean total number of N1 + N2 individual LNs dissected was 8.9 LNs in the thoracotomy group and 7.4 LNs in the conventional VATS group, while the number of individual N2 LNs dissected was 3.7 LNs in the thoracotomy group and 2.5 LNs in the conventional VATS group (26).
A previously reported overall upstaging rate for R-VATS lobectomies was 21% in a multi-center study by Park, while our overall upstaging rate was 30% (27). A comparative review published showed that the nodal upstaging rate for robotic-assisted resection (10.9%) appears to be superior to conventional VATS and similar to thoracotomy when analyzed by clinical T stage (28). Table 6 shows descriptive upstaging rates for open, conventional VATS, and R-VATS published in the literature (25,28-33). These upstaging rates compare unfavorably to our 8.2% upstaging rate for cN0 to pN1 and 8.2% upstaging rate for cN0 to pN2, with a combined cN0-to-pN1/N2 upstaging rate of 16.4%. That said, the highest level of cN0 to pN1/N2 upstaging that we found in the literature was reported by Watanabe and colleagues, with a 20% cN0-to-pN1/N2 upstaging rate for conventional VATS and 30% for open thoracotomy (31). However, in that series, the surgeons routinely performed bilateral mediastinal LN dissection for each of their unilateral lung resection cases.
The high percentage of overall pathologic upstaging is likely due to more complete mediastinal LN dissection than we would have been able achieve with either deliberate LN sampling only or else inadequate LN dissection due to limitations in visualization, instrumentation, or technical experience with the conventional VATS approach. However, several other factors, apart from use of the robotic surgical system, might also influence the higher percentage of nodal upstaging compared to the referenced literature, such as inclusion of patients with higher stage tumors (such as stages IIIA and higher), patients whose tumors required more extensive resection (such as chest wall or vertebral resection), patients with potentially more aggressive tumors (such as neuroendocrine carcinomas), and lack of preoperative mediastinal staging (such as by cervical mediastinoscopy or EBUS) for more locally advanced tumors.
Conclusions
Robotic-assisted pulmonary lobectomy is safe and effective, with perioperative outcomes that are comparable to those of conventional VATS lobectomy. Our perioperative outcomes are also comparable to the scarce data available for robotic-assisted lung surgery. Our numbers of N2 stations dissected and of total LNs analyzed as well as our upstaging rates demonstrate that hilar and mediastinal LN dissection during R-VATS lobectomy for lung cancer effectively facilitates detection of occult metastatic disease and is comparable, if not at times better, than historic data published for conventional VATS or thoracotomy approaches.
Acknowledgements
This research was supported by a 2013 Summer Scholarly Award to KL Rodriguez from the Scholarly Concentrations Program at the University of South Florida (USF) Health, Morsani College of Medicine.
Footnote
Lung Capacity After Lobectomy
Conflicts of Interest: EM Toloza and JP Fontaine have had financial relationships with Intuitive Surgical Corporation in form of honoraria as robotic thoracic surgery proctors and observation sites. This study was previously presented as an oral presentation at the Chest World Congress 2014 in Madrid, Spain, on March 22, 2014.
Ethical Statement: This study used a database protocol that was approved by our institution’s Scientific Review Committee (MCC #16512) and our university’s Institutional Review Board (IRB #Pro00002678), which waived informed consent for this retrospective study, which is considered as review of existing data.
Left Upper Lobectomy
References
- Rusch VW, Crowley J, Giroux DJ, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the N descriptors in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2007;2:603-12.
- Lee HS, Jang HJ. Thoracoscopic mediastinal lymph node dissection for lung cancer. Semin Thorac Cardiovasc Surg 2012;24:131-41. [Crossref] [PubMed]
- Gopaldas RR, Bakaeen FG, Dao TK, et al. Video-assisted thoracoscopic versus open thoracotomy lobectomy in a cohort of 13,619 patients. Ann Thorac Surg 2010;89:1563-70. [Crossref] [PubMed]
- Boffa DJ, Allen MS, Grab JD, et al. Data from The Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg 2008;135:247-54. [Crossref] [PubMed]
- Louie BE, Farivar AS, Aye RW, et al. Early experience with robotic lung resection results in similar operative outcomes and morbidity when compared with matched video-assisted thoracoscopic surgery cases. Ann Thorac Surg 2012;93:1598-604; discussion 1604-5. [Crossref] [PubMed]
- Ceppa DP, Kosinski AS, Berry MF, et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons Database analysis. Ann Surg 2012;256:487-93. [Crossref] [PubMed]
- Kent M, Wang T, Whyte R, et al. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg 2014;97:236-42; discussion 242-4. [Crossref] [PubMed]
- Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006;131:54-9. [Crossref] [PubMed]
- Travis WD. Advances in neuroendocrine lung tumors. Ann Oncol 2010;21 Suppl 7:vii65-71. [Crossref] [PubMed]
- Iyoda A, Makino T, Koezuka S, et al. Treatment options for patients with large cell neuroendocrine carcinoma of the lung. Gen Thorac Cardiovasc Surg 2014;62:351-6. [Crossref] [PubMed]
- Takagi H, Yamamoto H, Goto SN, et al. Perioperative results of robotic lung lobectomy: summary of the literature. Surg Endosc 2012;26:3697-9. [Crossref] [PubMed]
- Adams RD, Bolton WD, Stephenson JE, et al. Initial multicenter community robotic lobectomy experience: comparisons to a national database. Ann Thorac Surg 2014;97:1893-8; discussion 1899-900.
- Cao C, Manganas C, Ang SC, et al. A systematic review and meta-analysis on pulmonary resections by robotic video-assisted thoracic surgery. Ann Cardiothorac Surg 2012;1:3-10. [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Solaini L, Prusciano F, Bagioni P, et al. Video-assisted thoracic surgery (VATS) of the lung: analysis of intraoperative and postoperative complications over 15 years and review of the literature. Surg Endosc 2008;22:298-310. [Crossref] [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- Demmy TL, Nwogu C. Is video-assisted thoracic surgery lobectomy better? Quality of life considerations. Ann Thorac Surg 2008;85:S719-28. [Crossref] [PubMed]
- Louie BE. Robotic lobectomy - the future of minimally invasive lobectomy? Chin J Cancer Res 2013;25:1-3. [PubMed]
- Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg 2005;80:2051-6; discussion 2056. [Crossref] [PubMed]
- Osarogiagbon RU, Allen JW, Farooq A, et al. Objective review of mediastinal lymph node examination in a lung cancer resection cohort. J Thorac Oncol 2012;7:390-6. [Crossref] [PubMed]
- Watanabe A, Nakazawa J, Miyajima M, et al. Thoracoscopic mediastinal lymph node dissection for lung cancer. Semin Thorac Cardiovasc Surg 2012;24:68-73. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Swanson SJ, Herndon JE 2nd, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [Crossref] [PubMed]
- Hagan ME, Williams ST, Socci L, et al. Completing the audit cycle improves surgical standards in lung cancer. Why do some patients still not receive the best care? J Thorac Oncol 2013;8:779-82. [Crossref] [PubMed]
- D'Amico TA, Niland J, Mamet R, et al. Efficacy of mediastinal lymph node dissection during lobectomy for lung cancer by thoracoscopy and thoracotomy. Ann Thorac Surg 2011;92:226-31. [Crossref] [PubMed]
- Denlinger CE, Fernandez F, Meyers BF, et al. Lymph node evaluation in video-assisted thoracoscopic lobectomy versus lobectomy by thoracotomy. Ann Thorac Surg 2010;89:1730-5. [Crossref] [PubMed]
- Park BJ. Robotic lobectomy for non-small cell lung cancer (NSCLC): Multi-center registry study of long-term oncologic results. Ann Cardiothorac Surg 2012;1:24-6. [PubMed]
- Wilson JL, Louie BE, Cerfolio RJ, et al. The prevalence of nodal upstaging during robotic lung resection in early stage non-small cell lung cancer. Ann Thorac Surg 2014;97:1901-6. [Crossref] [PubMed]
- Sugi K, Kaneda Y, Esato K. Video-assisted thoracoscopic lobectomy achieves a satisfactory long-term prognosis in patients with clinical stage IA lung cancer. World J Surg 2000;24:27-30; discussion 30-1. [Crossref] [PubMed]
- Boffa DJ, Kosinski AS, Paul S, et al. Lymph node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann Thorac Surg 2012;94:347-53. [Crossref] [PubMed]
- Watanabe A, Koyanagi T, Ohsawa H, et al. Systematic node dissection by VATS is not inferior to that through an open thoracotomy: a comparative clinicopathologic retrospective study. Surgery 2005;138:510-7. [Crossref] [PubMed]
- Verhagen AF, Schoenmakers MC, Barendregt W, et al. Completeness of lung cancer surgery: is mediastinal dissection common practice? Eur J Cardiothorac Surg 2012;41:834-8. [Crossref] [PubMed]
- Choi MS, Park JS, Kim HK, et al. Analysis of 1,067 cases of video-assisted thoracic surgery lobectomy. Korean J Thorac Cardiovasc Surg 2011;44:169-77. [Crossref] [PubMed]